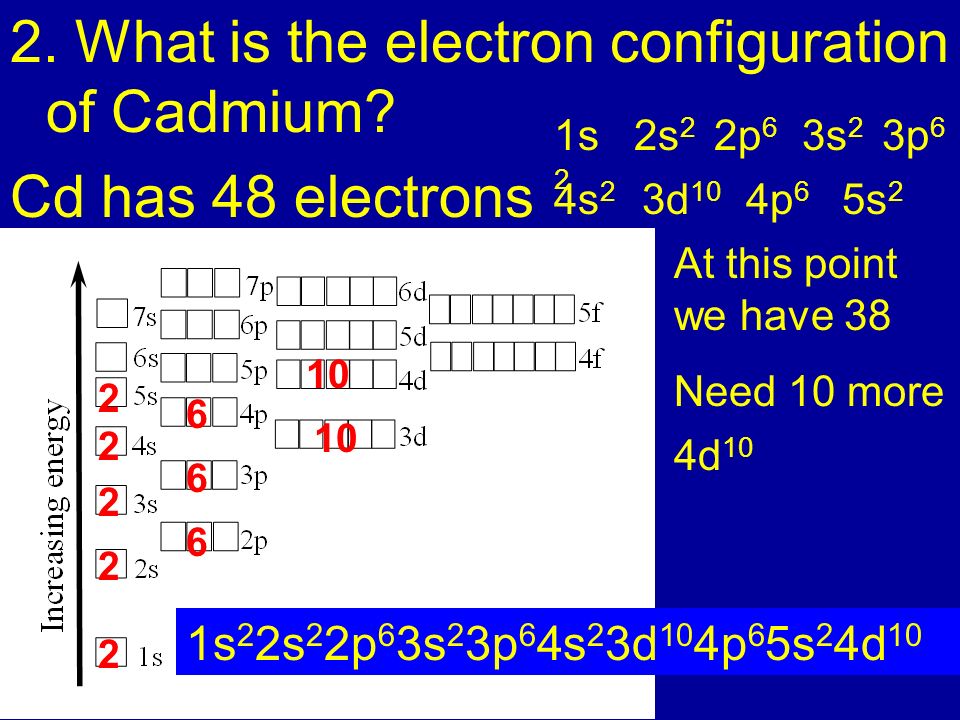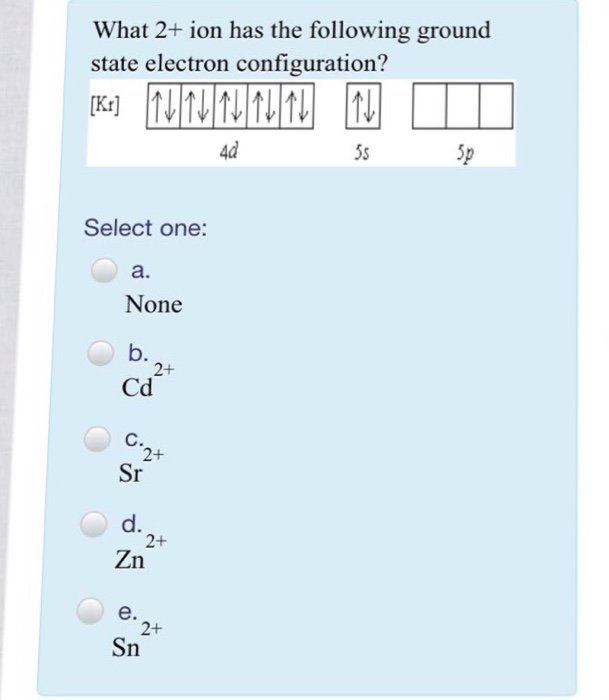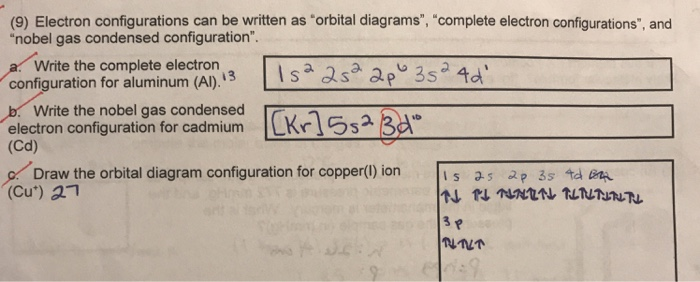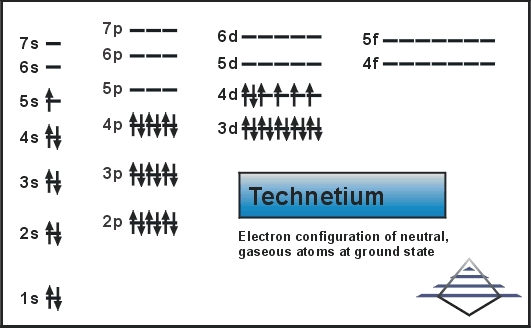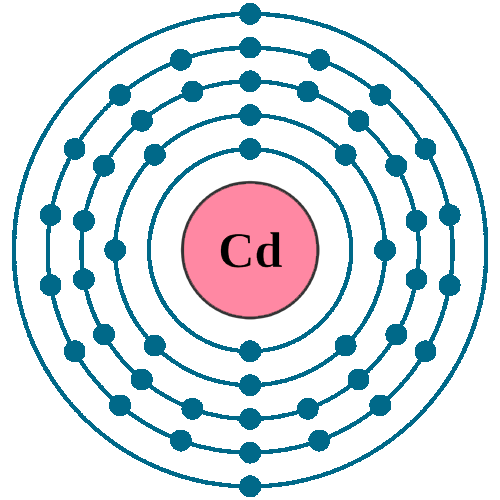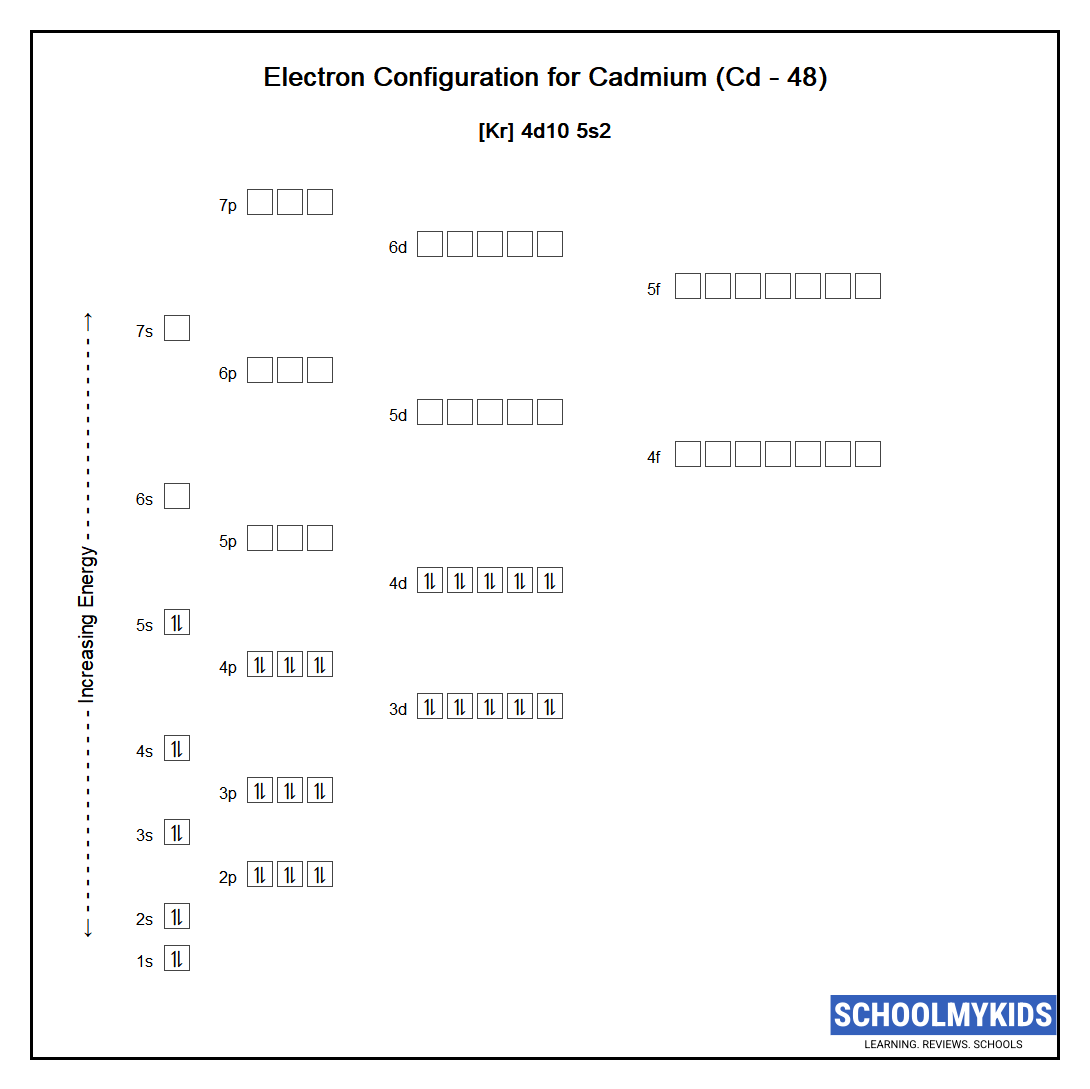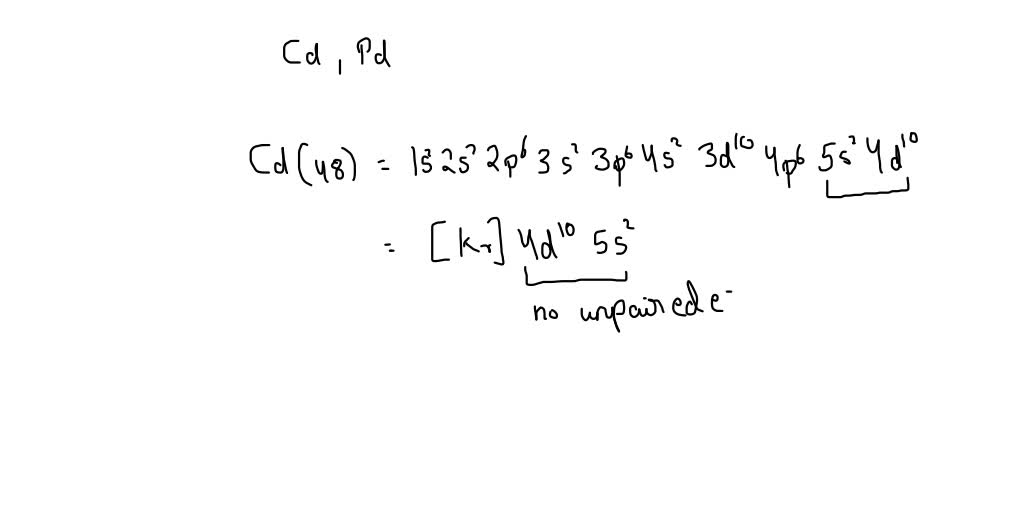
SOLVED: Predict the ground-state electron configuration of the following atoms indicate if they are expected to be paramagnetic or diamagnetic. Cadmium (electron configuration) Lead (noble gas shorthand notation)

Write the condensed ground-state electron configuration for Cd2+. Is it paramagnetic or diamagnetic? | Homework.Study.com
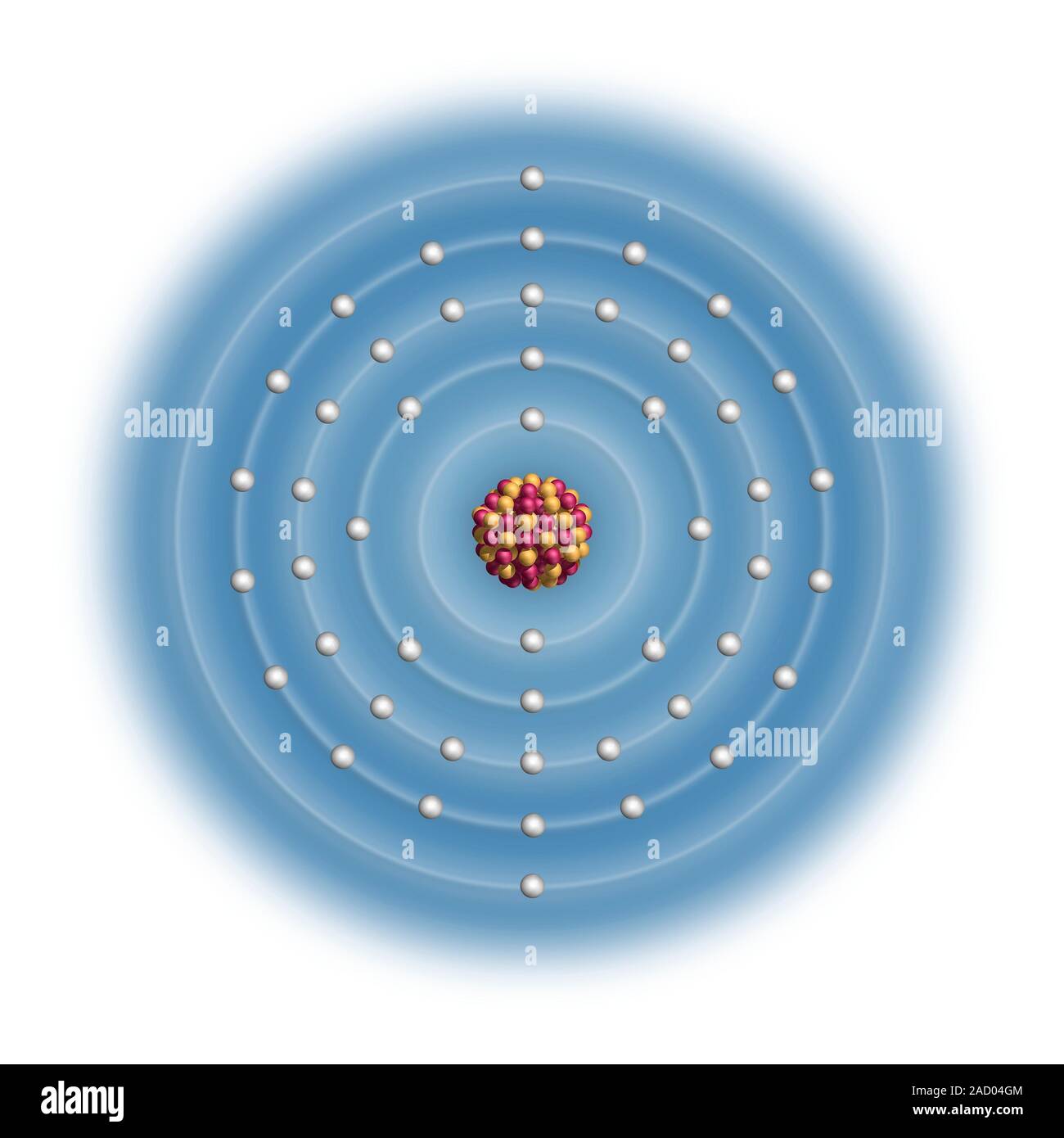
Le cadmium (Cd). Schéma de la composition nucléaire et configuration électronique d'un atome de cadmium-114 (numéro atomique : 48), l'isotope le plus courant de Photo Stock - Alamy


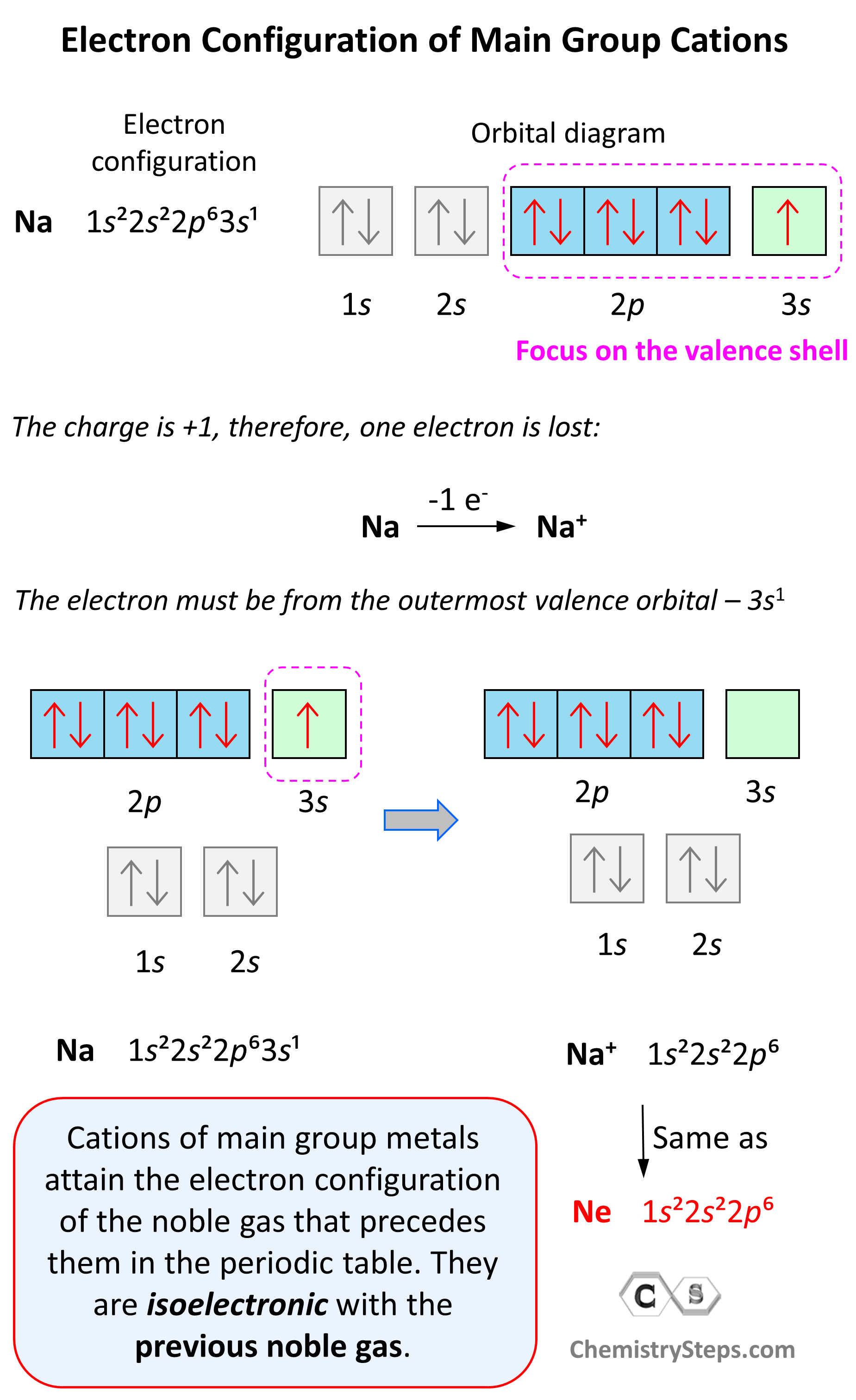
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)